balanSys Revision
MATHYS
balanSys Revision
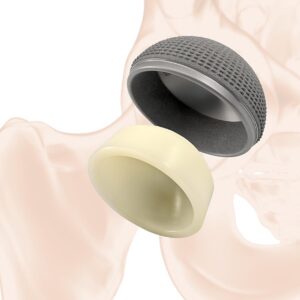
balanSys REV is a system for total knee replacement. The design is based on bone-oriented resurfacing, and its geometries are identical to those of the bal-anSys BICONDYLAR system for primary implantation. Offering the additional advantage of allowing combination of the two systems in situations with bone defects but without ligament instability, it enables loss of healthy bone to be minimised.
balanSys REV is a versatile, stable and proven revision implant system with 15 years of clinical experience and demonstrated improvement of knee function.1,2
The implant system was developed in cooperation with a European surgeon team. The system permits replacement of previously implanted components with minimal bone loss for optimal restoration of bone deficiencies and joint lines. The surgical technique allows adequate coverage of the soft-tissues and balancing of the ligaments for stability of the revision implant. The resurfacing geometry of the implant is based on the design of the balanSys BICONDYLAR implant, rendering it compatible with the balanSys knee system for primary implantation. Precision instruments allow intra-operatively reproducible orientation of the stems. All bone cuts are template-guided for reliable resections.
The femoral and tibial implants made from a cast cobalt-chrome alloy are cemented, and optionally the same type of non-cemented stem can be fitted to the femoral and tibial components alike. The stems come in 12 diameters and three lengths as a straight and a 4-mm-offset version. 5-mm and 10-mm augments are available for the femoral and tibial components.
Update: The Enovis and Mathys product range is now exclusively available through LimaCorporate in the UK. Osteotec remains the distributor for Enovis and Mathys customers in Ireland (Republic of Ireland and Northern Ireland) and the Nordics (Norway, Sweden, Denmark, and Finland).
Enquire
For further information, questions regarding this product, or to discuss alternative solutions, please get in touch with your local Sales Specialist or our Head Office using the form or the contact details at the bottom of the page.
Stay in touch
NEWSLETTER
Sign up to receive email updates on new product announcements, insights on surgical techniques from surgeons, specialists, and sales representatives and industry trends, such as changes in regulations and new research findings.