aneXys
MATHYS
aneXys
Cup system
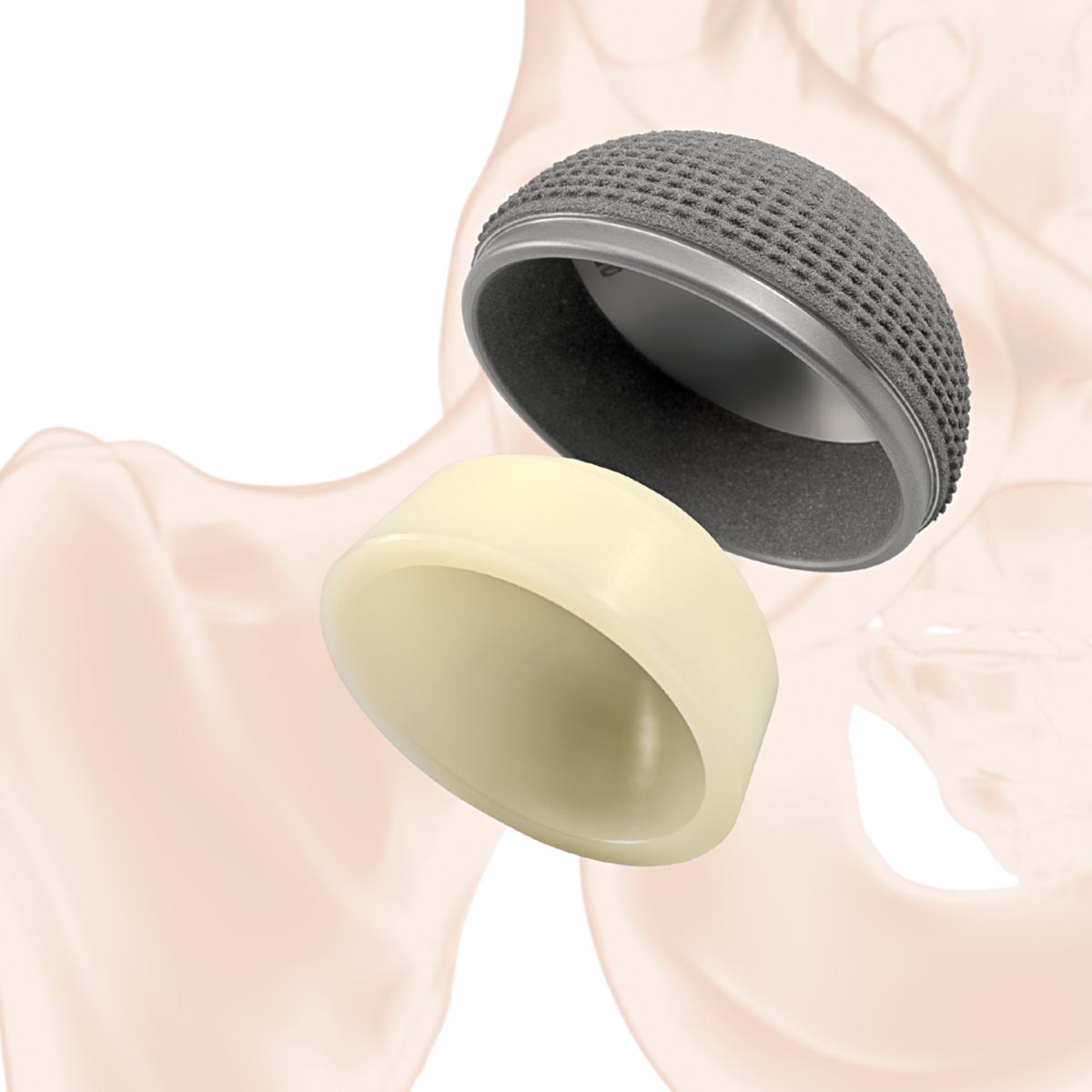
The aneXys cup system is a modular uncemented cup option for hip replacement surgery. It offers a wide range of component combinations to accommodate the individual patient anatomical requirements and functional demands.
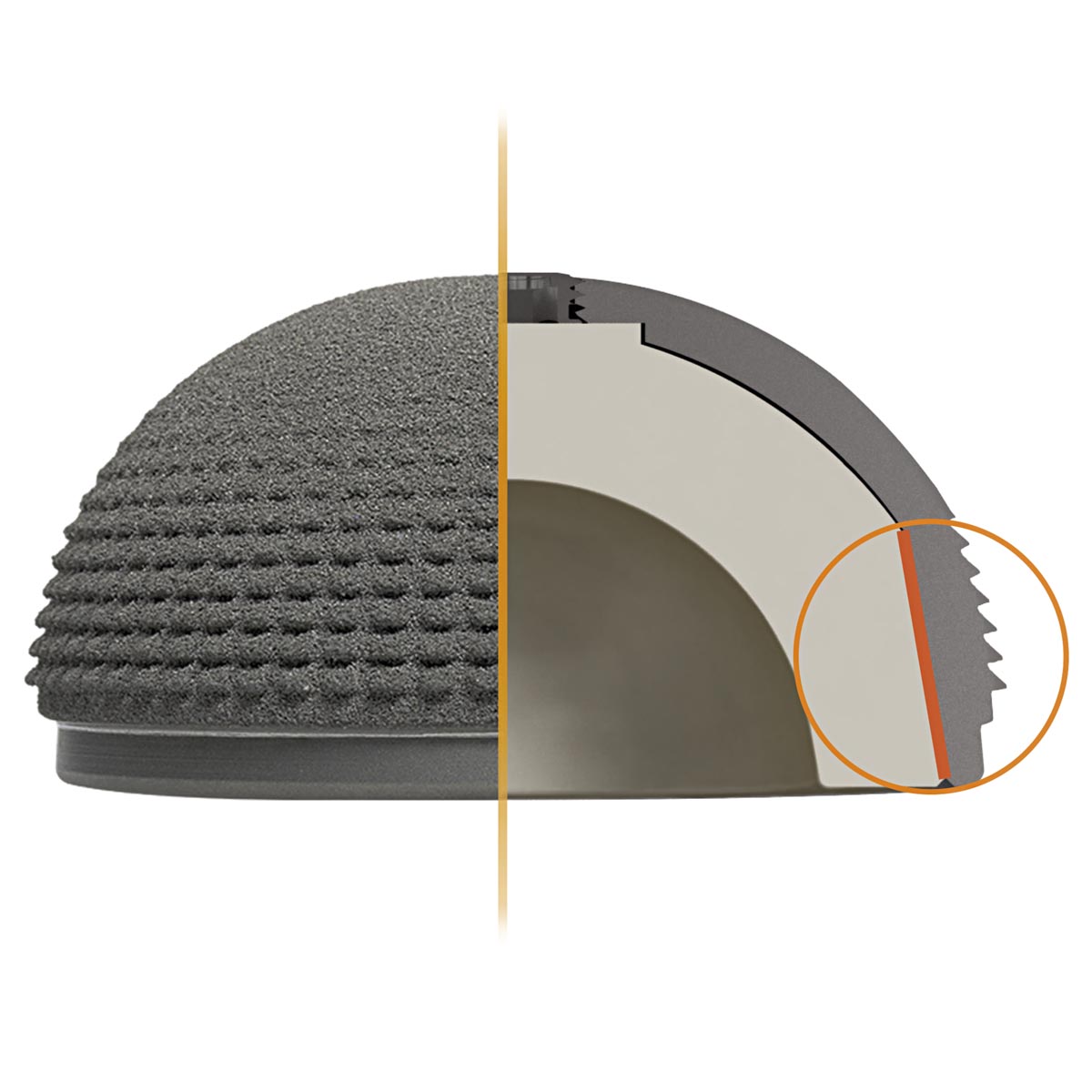
The equatorial oversizing, in combination with the macro-structured, titanium-coated and porous surface allows the cup to achieve high mechanical primary stability. The porosity of the coating is up to 50%, thereby providing good conditions for the biological integration of the implant in the pelvic bone.
The modular inlays are made of vitamys or ceramys. vitamys is a highly cross-linked polyethylene stabilised with vitamin E (VEPE). ceramys is a dispersion ceramic (ATZ) made of a homogeneous mixture of 80% yttriumoxide stabilized zirconia and 20% alumina and contains no other additives.
Intraoperative flexibility in the selection of the cup (aneXys Flex, aneXys Uno, aneXys Cluster or aneXys Multi) and inlay (vitamys, ceramys) in combination with a well-designed, modular instrument set allows an efficient workflow with all commonly used surgical approaches.
Update: The Enovis and Mathys product range is now exclusively available through LimaCorporate in the UK. Osteotec remains the distributor for Enovis and Mathys customers in Ireland (Republic of Ireland and Northern Ireland) and the Nordics (Norway, Sweden, Denmark, and Finland).
Enquire
For further information, questions regarding this product, or to discuss alternative solutions, please get in touch with your local Sales Specialist or our Head Office using the form or the contact details at the bottom of the page.
Stay in touch
NEWSLETTER
Sign up to receive email updates on new product announcements, insights on surgical techniques from surgeons, specialists, and sales representatives and industry trends, such as changes in regulations and new research findings.