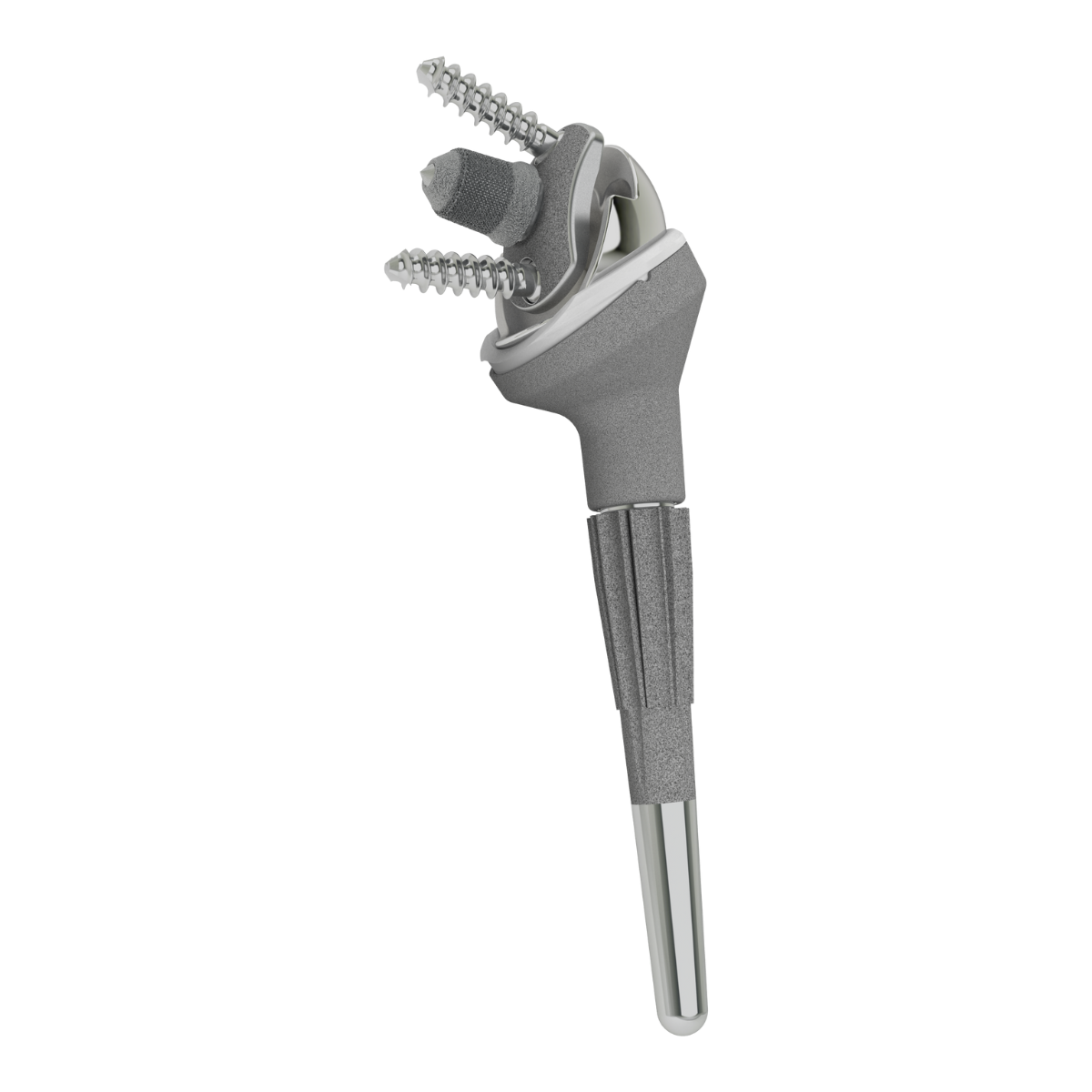
SMR TT Hybrid Glenoid
LimaCorporate
SMR TT Hybrid Glenoid
The SMR TT Hybrid Glenoid is the first convertible 3D Printed* Hybrid Glenoid. The central peg is made in LimaCorporate’s patent technology.
*Trabecular Titanium.
BENEFITS
- Soft Tissue management – The wide range of sizes, mismatch options and thicknesses make SMR TT Hybrid Glenoid appropriate for soft tissue balancing.
- Convertible glenoid – SMR TT Hybrid Glenoid is invertible to reverse without the central peg removal.
- Reliable fixation – The 3D printed Trabecular Titanium central peg enhances primary and biological fixation. Poly peripheral pegs are also cemented.
Enquire
For further information, questions regarding this product, or to discuss alternative solutions, please get in touch with your local Sales Specialist or our Head Office using the form or the contact details at the bottom of the page.
Stay in touch
NEWSLETTER
Sign up to receive email updates on new product announcements, insights on surgical techniques from surgeons, specialists, and sales representatives and industry trends, such as changes in regulations and new research findings.